Dana Farber Researchers use Untargeted Metabolomics to Identify New Metabolic Mechanisms in Pancreatic Cells
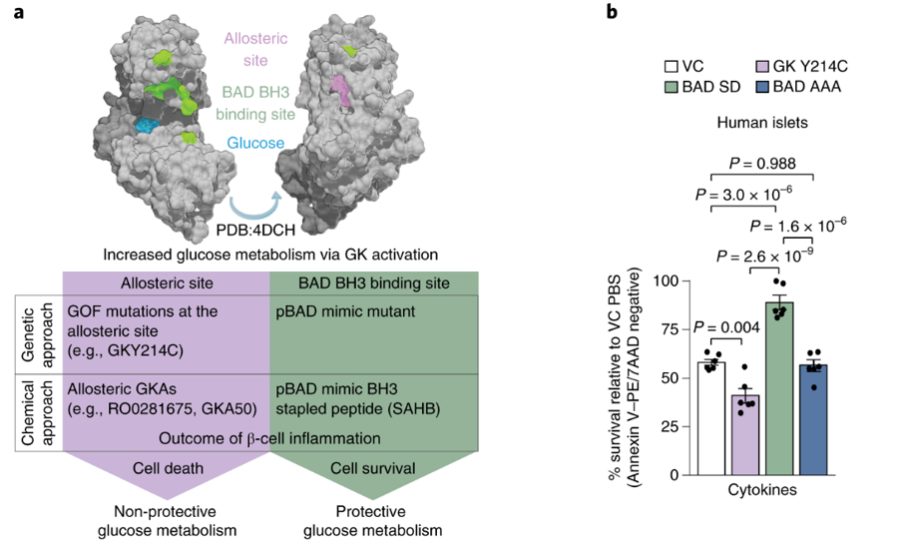
Diabetes is an incurable metabolic disease that affects over 500 million people worldwide. Despite a large body of evidence suggesting that chronic inflammation exacerbates and potentially causes diabetes, the factors that contribute to pancreatic β-cells sensitivity to inflammation are poorly understood.
In this study, published in Nature Metabolism, researchers at Dana Farber Cancer Institute led by Accalia Fu and Nika Danial examined the metabolic consequences of inflammation in isolated human pancreatic islets. This uncovered the surprising observation that activation of glucose metabolism through glycolysis could block the negative effects of inflammation, ultimately leading to increased β-cell survival. To understand the mechanisms underlying how glucose mediates this survival benefit, the researchers worked with General Metabolics to profile the metabolome of pancreatic islets under various states of inflammation. Taking advantage of General Metabolics’ untargeted metabolomics platform, the researchers found that β-cell survival was associated with significant alterations in the urea cycle , providing an initial clue to the metabolic mechanism.
To further characterize the mechanism, the researchers utilized proteomics, targeted metabolomics, and metabolic tracer studies to assess the role of the urea cycle in protection from inflammation-induced cell death. They demonstrated that the cell-protective activation of glucokinase leads to activation of pyruvate carboxylase, which supports carbon flux into the urea cycle and ultimately leads to a reduction in pro-inflammatory nitric oxide and increased urea production.
This study demonstrates the value of a broad, untargeted profiling approach to uncover new biological mechanisms, and identifies a metabolic phenotype that may have clinical relevance for diabetes treatment. General Metabolics offers a fast, efficient, and affordable way to identify sites of metabolic regulation and to generate hypotheses that enable the design of mechanism-focused follow-up experiments.
If you would like to learn more about how our high-throughput untargeted metabolomics platform can support your research, please contact us.